Our Pipeline
Seed-and-Boost Strategy Enables Effective Targeting of Multiple Solid Tumors While Maximizing Patient Coverage Across Global Populations
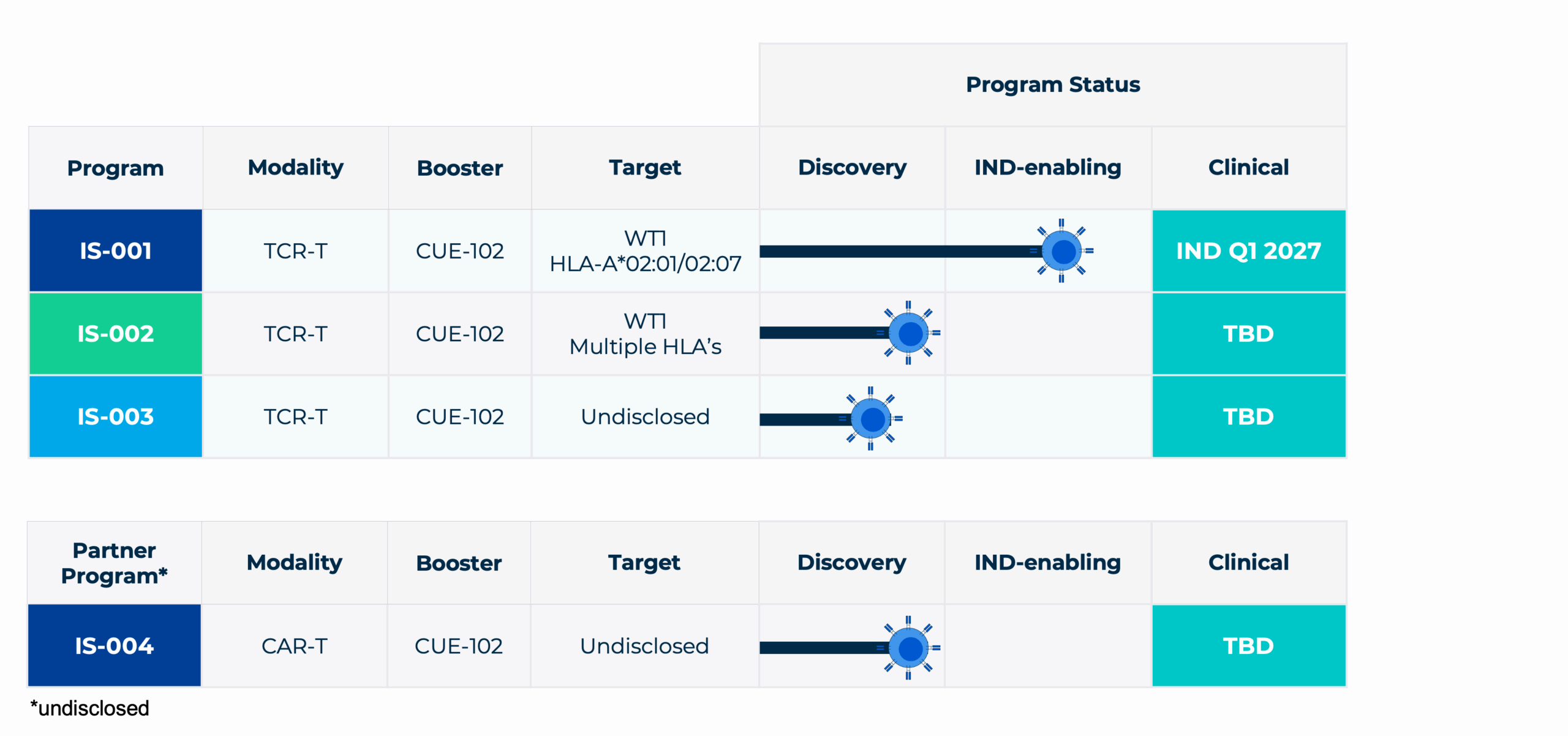
Our lead program IS-001 combines WT1 TCR-T cells with CUE-102:
WT1 is a clinically validated, high-impact tumor antigen broadly expressed across solid and hematological malignancies, with minimal off-target toxicity. Recognized as a top-priority target for cancer immunotherapy by the National Cancer Institute, WT1 represents one of the most promising opportunities for TCR-T cell therapies.
CUE-102 is a clinically validated molecule, specifically engineered to engage and expand WT1-specific T cells in vivo, while minimizing the severe toxicities associated with non-specific IL-2 cancer immunotherapies. CUE-102 has demonstrated favourable safety and tolerability in a Phase 1 across multiple WT1-positive tumor indications.
WT1-directed TCR-T cells, in combination with CUE-102, represent a superior cell therapy approach that enables minimal T-cell dosing while promoting selective activation, in vivo expansion, and durable antitumor efficacy. Our first clinical program will target WT1-positive solid tumors across multiple indications.
